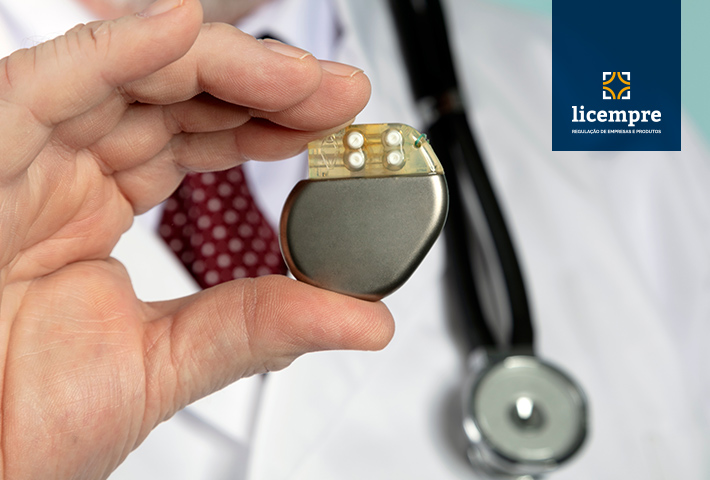
Product registration is the activity that guarantees the safety of products offered to consumers. ANVISA, as the body responsible for the registration process of health products, supervises, approves, and guarantees the safety of the product until it reaches the consumer. Health equipment such as a pacemaker is a mandatory equipment registration since they must guarantee the safety of the user by not putting him at risk or exposed to any problem. In this case, because it is a product of extreme care, the regulatory body requires that all regulatory standards to be due, both by the manufacturer, importer, and the product. Next, you will understand how the pacemaker registration process is carried out and the importance of having regulatory advice in carrying out the process.
What is a pacemaker?

The pacemaker is a small device placed close to the heart through surgery. Considered an implant, it is recommended for patients who have been diagnosed with heart failure. The device is responsible for correcting the heart rate of a patient who has some type of deficiency in this regard. The pacemaker can be implanted for a period or permanently depending on the patient’s condition.
How to regulate the pacemaker?
 Now that you know what the pacemaker is, you will understand what the registration process is like. The pacemaker fits in the regularization of medical equipment being regulated first by Inmetro and only afterwards the product can be registered by Anvisa – National Health Surveillance Agency. It needs to be regulated to be commercialized.
Now that you know what the pacemaker is, you will understand what the registration process is like. The pacemaker fits in the regularization of medical equipment being regulated first by Inmetro and only afterwards the product can be registered by Anvisa – National Health Surveillance Agency. It needs to be regulated to be commercialized.
As established in art. 12 of Law 6,360, of September 23, 1976, no product of interest to health, whether domestic or imported may be industrialized, exposed for sale or delivered for consumption in the Brazilian market before being registered with the Ministry of Health. indicated in § 1 of Art. 25 of the said Law, which although exempted from registration, are subject to the Health Surveillance regime (they are registered products).
What happens if I do not regularize the product?
 Given this, we must remember that every product to be regularized, must first have the company registration, that is, no importer or manufacturer is authorized to sell their product if it does not previously comply with all the company registration criteria presented by Organs regulatory agencies.
Given this, we must remember that every product to be regularized, must first have the company registration, that is, no importer or manufacturer is authorized to sell their product if it does not previously comply with all the company registration criteria presented by Organs regulatory agencies.
Failure to comply with the determinations provided for in the sanitary legislation characterizes a violation of the Federal Sanitary Legislation, and the offending company is under penalties,within the administrative scope, provided for in Law No. 6,437, of August 20, 1977, without prejudice to civil or penal sanctions. applicable. In the legal sphere, the Legal and Technical Responsible Persons are responsible for the acts of infraction practiced by the company, according to the infractions and sanctions provided for in art. 273 of Decree Law No. 2,848, of December 7, 1940 (Penal Code – Chapter III: Crimes against Public Health).
You can read more about business registration on our business registration page. Once the company has been regularized, everything will be ready for product registration. For this, the manufacturer needs to present all data and information about the product including composite materials in the composition, handling, side effects, useful life, Inmetro certificate, among other information that will be presented to the agency. The information goes to Anvisa to analyze and approve, and the product can only be approved after taking all tests and standards established by ANVISA.
Registration must be requested by submitting, to Anvisa, a request for registration or registration request, composed of documents and information indicated in DRC Anvisa No. 185/01 and other pertinent legislation, thus constituting a documentary process. The registration request is based on RDC Anvisa nº 24/09. The forwarded process is analyzed by Anvisa’s technical staff, who will deliberate on the granting of the request, and may request additional information and documents, when necessary.
After the process is completed, the manufacturer or importer is notified and only then will he be able to market his product.
Why to regularize with Licempre?
 The processes for regularizing a product, especially health equipment that requires greater care in relation to safety, take into account all bureaucratic processes. Thus, having experienced professionals, who already know about the whole process, makes all the difference in terms of time, price, and security. Talk to a Licempre specialist and register securely.
The processes for regularizing a product, especially health equipment that requires greater care in relation to safety, take into account all bureaucratic processes. Thus, having experienced professionals, who already know about the whole process, makes all the difference in terms of time, price, and security. Talk to a Licempre specialist and register securely.


