Difference between Registration and Notification of Health Products at Anvisa: Understand the Processes and Rules
November 11, 2024
Regulating medical materials and equipment is essential in the health sector to guarantee product safety and efficacy. In Brazil, Anvisa establishes the requirements necessary for these products to be authorized for sale, classifying them according to the risks they pose to the health of the user, patient, operator, or third parties involved. Companies wishing to register or notify medical products must follow a specific process depending on the product’s risk class.
Risk classification by Anvisa
- Class I: Low risk to individuals and public health
- Class II: Medium risk to the individual and/or low risk to public health
- Class III: High risk to the individual and/or medium risk to public health
- Class IV: High risk to the individual and high risk to public health
Class I and II products are generally subject to the notification process, while those classified as Class III and IV require formal registration due to their greater risk.
In addition to classification by risk, Anvisa also uses specific rules to classify 22 health products. These rules obey the indication and purpose of use of each product.
- What is Health Product Notification?
Product Notification is a simpler and faster procedure applied to products classified as Risk Classes I and II, which present a low to medium health risk. This process allows companies to launch products without facing the same stringent requirements as higher-risk products.
- Examples of Notifiable Products: Risk Class I products include masks, disposable gloves, and aprons. These items are widely used and offer low risk to users, facilitating their authorization process.
- Notification process: To notify Anvisa about a product, the company must send Anvisa a communication containing basic information about the product, such as its composition, purpose, and manufacturer’s data. After this notification, the product can be marketed with Anvisa’s authorization, allowing it to enter the market quickly.
2. What is Health Product Registration?
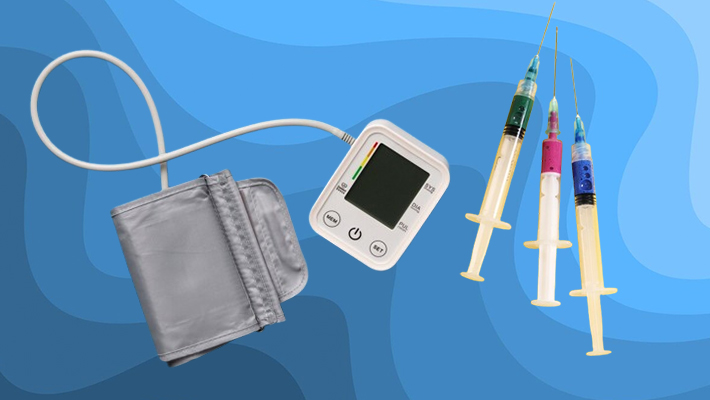
Product Registration is a more detailed process necessary for items that present a high risk to health and fall into Risk Classes III and IV. These products require in-depth analysis by Anvisa to guarantee their safety and efficacy.
- Examples of Registrable Products: Products such as glucometers, pressure gauges, and syringes are classified as Risk Classes III and IV, requiring careful evaluation to ensure their safe and efficient use.
- Registration process: Registration requires thorough documentation, including technical reports, safety and efficacy studies, and a detailed description of the product’s intended use. Anvisa assesses all the documentation before granting marketing authorization.
Summary of Risk Classes for Health Products
- Class I (Notification): Low-risk products that follow a simplified process. Examples: masks, disposable gloves, aprons.
- Class II (Notification): Medium-risk products that also follow the notification process. Examples: thermometers and some types of probes.
- Class III and IV (Registration): High-risk products that require a more detailed assessment and registration process. Examples: glucometers, pressure gauges, syringes.
What is the best option for your product?
The choice between Notification and Registration of Health Products depends on the product’s risk class. Class I and II products go through a simpler notification process, while Class III and IV require registration, which is more detailed and requires careful analysis by Anvisa.
At Licempre, we offer complete consultancy to help companies carry out the Notification or Registration of Health Products with Anvisa. Our consulting ranges from preparing documentation to ongoing support in complying with regulatory standards, ensuring that your products reach the market safely and in compliance.
Rely on Licempre for the fast and safe release of your health products!



